Technology & Products
[Battery Glossary] Sulfide Solid Electrolyte, All-Solid-State Battery
2025.12.18
|
[Battery Glossary] answers the questions related to batteries with key term explanation. From fundamental battery principles, manufacturing processes to emerging next-generation technologies, [Battery Glossary] makes battery concepts easy to understand. |
Sulfide Solid Electrolyte
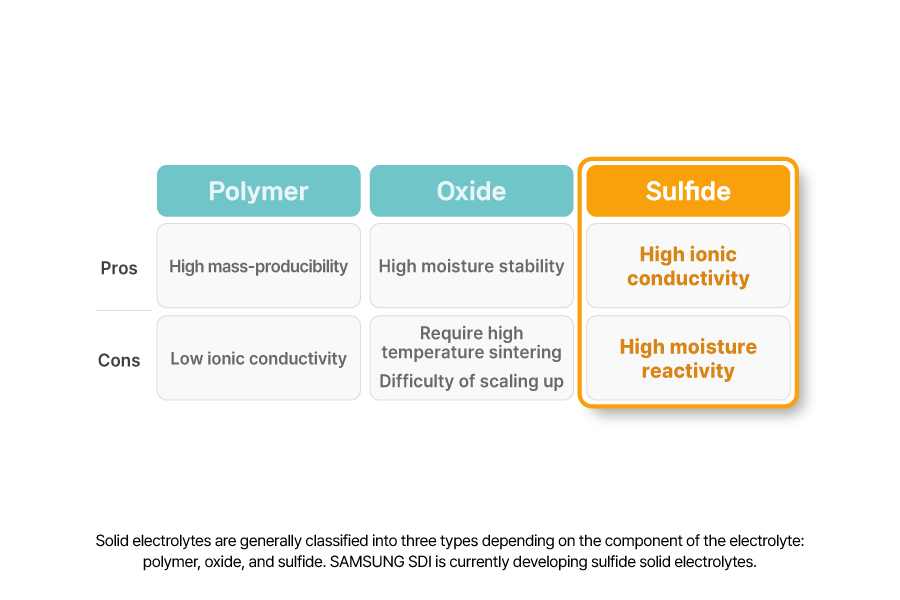
All-solid-state batteries use a solid electrolyte and are generally classified into three types depending on the component of the electrolyte: polymer, oxide, and sulfide. Among then, sulfide solid electrolytes—composed primarily of lithium-sulfur—are known to have the highest ionic conductivity to date. However, they are usually susceptible to moisture, making it critical for battery manufacturers to prevent any water ingress during production.
All-Solid-State Battery
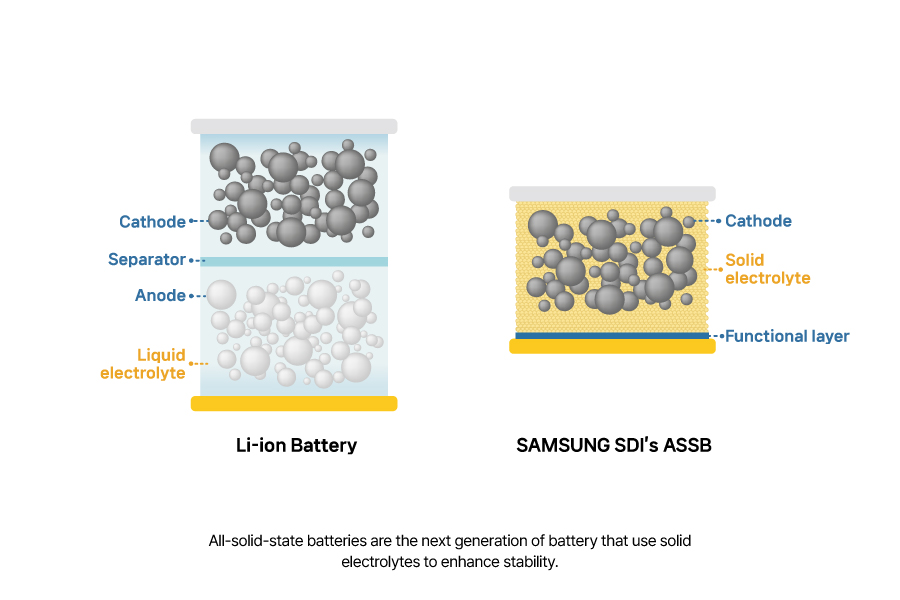
All-solid-state batteries are the next generation of battery that use solid electrolytes to enhance stability. Conventional lithium-ion batteries use a separator as a physical barrier between the cathode and anode, but if the separator is torn or punctured, the electrodes can come into contact, causing a short circuit. Since the organic solvents are typically made of flammable liquid electrolytes, this can result in fires. In contrast, all-solid-state batteries use solid electrolytes with non-flammable organic solvents, significantly lowering fire risk. They also have a higher energy density by using high-capacity lithium metal instead of graphite or silicon.
SAMSUNG SDI is developing all-solid-state batteries featuring an anodeless structure. An anodeless structure refers to a structure in which there is no anode when the cell is first manufactured, but during the charging process, lithium ions from the cathode migrate and form a layer that functions as the anode. One of the key features of SAMSUNG SDI’s all-solid-state battery is that there is a functional layer that controls the uniform formation of a layer.
