Technology & Products
[1-Minute Battery] Why Aluminum for the Cathode and Copper for the Anode?
2025.05.13
|
[1-Minute Battery] provides easy and quick explanations from simple curiosities about batteries to insightful questions! |
Both anodes and cathodes in Li-ion batteries are made by coating a current collector with a slurry of active materials, conductive additives and binders. A current collector is an ultra-thin metallic foil, measured in micrometers ㎛(1×10-6m), which acts as a pathway for electrons to travel and help keep the structure of the coating layer more robust.
Typically, Li-ion batteries use aluminum as the current collector for the cathode and copper for the anode. But have you ever wondered why these specific materials are used?

[Wouldn’t it be much easier to use the same materials for both the cathode and anode?]
The current collector’s primary role is to transport electrons generated during the battery’s charge and discharge cycles to external circuits, or to deliver electrons to the electrode’s active material. Therefore, the metal foil used as the current collector must have high electrical conductivity. For high energy density and high capacity, batteries must also be thin, lightweight, and cost-effective for business viability.
Among major metals, copper offers the second highest conductivity after silver. It can be easily processed into thin sheets and is relatively low in density, making it a highly suitable for current collectors.
* Order of electrical conductivity: Silver > Copper < Gold < Aluminum > Magnesium > Zinc > Nickel > Iron > Lead
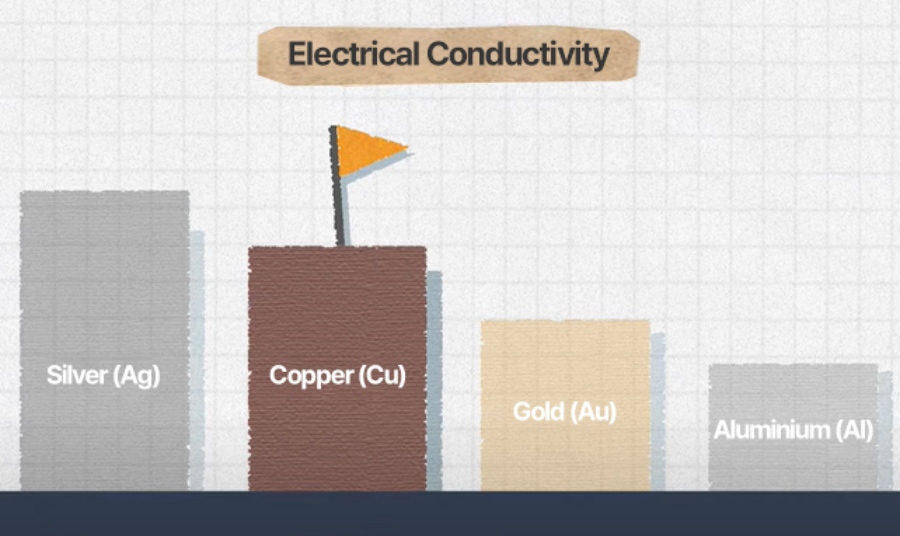
[While gold takes the top spot in the Olympics, silver actually leads in electrical conductivity—followed by copper and gold.]
Then why do we use aluminum—not copper—for the cathode current collector? And conversely, although aluminum is cheaper than copper, why can’t we use aluminum as the anode current collector?
The reason lies in the electrochemical properties of the materials. The anode operates at a high voltage, requiring the collector to be stable at high voltages (above 4V), and conversely, the cathode operates at a low voltage, requiring the collector to be stable at low voltages (below 0.01V).
Copper undergoes an electrochemical reaction (oxidation) with lithium at voltages above 3.385V, making it unsuitable as a cathode current collector. On the other hand, aluminum reacts (reduction) with lithium at low voltages to form alloys, making it unsuitable as an anode current collector.
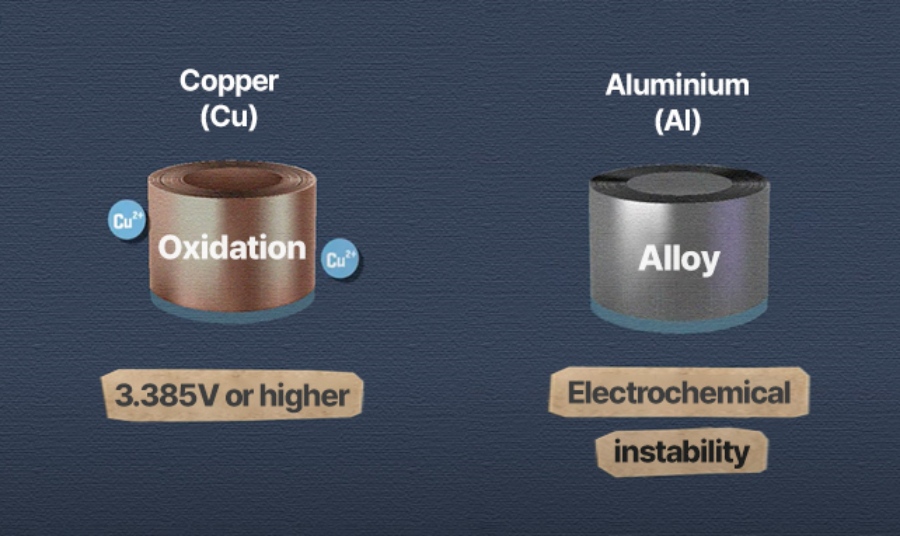
[Copper can’t be used on the cathode because it oxidizes at high voltage, and aluminum can’t be used on the anode because it forms alloys at low voltage.]
In short, cathode current collectors must be stable at oxidation potentials (above 4V). Anode current collectors must be stable at reduction potential (below 0.01V). Interestingly, aluminum itself is inherently prone to oxidation. However, it naturally forms a protective Al2O3 layer when exposed to air, which prevents further oxidation and makes it viable for cathode applications.
As for alternative materials for anode current collectors, metals such as nickel and cobalt could theoretically be used since they do not easily form alloys with lithium at low voltages. However, when factors like electrical conductivity, mechanical processability, thermal stability, and cost are considered, copper emerges as the best overall choice and is therefore widely used.
In conclusion, although the roles of cathode and anode current collectors are similar, different materials are used to optimize the performance and stability of Li-ion batteries. <1-Miniute Battery> will return soon with even more interesting topics!
