Technology & Products
[Battery101] Cathode Materials That Determine the Power of Li-ion Battery
2024.06.28
|
101[wʌ́nouwʌ́n] means basic knowledge of a topic or collection of introductory materials to a topic. Our Battery 101 series talks about all things battery: the history, technical aspects (basic principles and mechanisms), industrial aspects (IT, electric vehicles, ESS, etc.), and next-generation technologies that SAMSUNG SDI will innovate while opening up its future. Batteries have infinite potentials that exceeds our wildest imagination. Through Batteries 101 series, you will have a chance to see the entire spectrum of the battery's possibilities and to conjure SAMSUNG SDI’s pivotal role in it. |
It’s lithium metal oxides, not lithium
Lithium-ion batteries use lithium as a cathode materials. The ions (electrically neutral atoms that have lost or gained electrons) are involved in generating electrical energy, rather than lithium metal itself. A battery is charged when lithium ions and electrons move towards the anode, and it is discharged when lithium ions and electrons return to the cathode. That is why the cathode is often called the “home” of lithium ions.
[Configuration of the cathode]
The cathode is a mixture of active materials, conductive additives, and binders coated on an aluminum plate. As a substrate, aluminum serves the role of collecting and moving electrons only and is not involved in the generation of electrons. The active material is the main source of electricity generation. It is lithium that determines the capacity and voltage of the battery.
Lithium is regarded as an expensive and precious material among raw materials for batteries, and that is why it is often called “white oil.” Lithium is one of the lightest alkali metals, and its atomic number is 3. Because it is structured to lose electrons easily, lithium is highly reactive and has a very low density. It is not easy to transfer and store lithium by itself as it reacts with water and air. This is why lithium metal oxides are used for lithium-ion batteries, instead of pure lithium. Lithium is bound to oxygen as an oxide, which makes it easier to use it as an active material.
Cathode active materials (CAMs) have different properties depending on the types of metals
The lithium metal oxide as CAMs carry different properties depending on the metals applied. For instance, nickel (Ni) enhances capacity, manganese (Mn) and cobalt (Co) increase safety, and aluminum (AI) improves output characteristics.
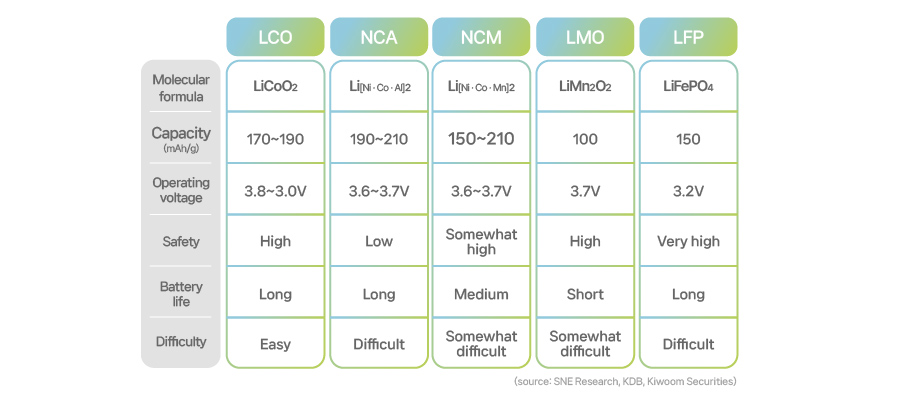
[Types and properties of CAMs for lithium-ion batteries]
Battery companies mix those metals in active materials to produce five types of lithium-ion batteries: lithium cobalt oxide (LCO), lithium manganese oxide (LMO), and lithium iron phosphate (LFP) which are some of the first inventions, along with lithium nickel cobalt manganese oxide (NCM) and lithium nickel cobalt aluminum oxide (NCA), which are variations of LCO where some active materials are replaced with nickel and aluminum with cobalt as a principle element.
Let’s begin with LCO, the first generation of cathode active materials. Professor Goodenough, Nobel Prize winner in Chemistry, unveiled LCO for the first time in his paper published in 1980. LCO is a compound of lithium, cobalt, and oxygen particles. LCO gained sky-rocketing popularity after it was used in portable electronic devices in the early 1990s.
What is the first thing that pops into your head when you think of cobalt? Many think of a blue sea in cobalt blue. To the surprise of many, cobalt is actually a silver-white metal. It turns blue or red when it reacts with other elements making it good coloring for ceramics, glass, and tiles for centuries. These days, cobalt has been drawing attention of many industries as it enables high-strength in alloys for powerful magnets, high-speed cutting tools, jet engines, and gas turbines. Cobalt is a rigid and ferromagnetic substance (a property that magnetizes without any external magnetic field). Cobalt does not react with air and water but slowly dissolves in diluted acids. It is in stark contrast to lithium which reacts with air and water quite violently. Therefore, the optimal mixture of lithium and cobalt can result in batteries that can control the risk of explosion and corrosion. This is also why they are widely used in electronic devices like smartphones.
However, LCO poses limitations in increasing output and cobalt. It also accounts for a high share of battery manufacturing costs. This is because cobalt cannot be mined in its pure state and is mostly obtained by refining byproducts of mined copper or nickel. Given that about 70% of cobalt produced across the world comes from the Democratic Republic of Congo, unstable cobalt supply chains are a big issue. Thus, there was a need to reduce the content of cobalt and substitute it with other materials so that batteries can expand their applications like electronic tools and electric vehicles while improving higher capacity and improved outputs and safety levels.
Nickel was the industry’s first choice. Nickel is a robust silver-white metal abundant on earth, and it boasts high ductility. It belongs to the iron group along with iron and cobalt, and therefore has similar properties. Nickel does not react with air, and does not oxide easily. Even when oxidation takes place, the metal rarely rusts as its dense membranes protect the inside, which is why nickel is commonly used in alloys and plating. When mixed with lithium, nickel can work to reduce the high reactivity of lithium. In addition, nickel not only has a higher global production size than cobalt but also is less expensive. By raising the nickel ratio, it is possible to maintain the performance while cutting down the price. NCM and NCA, commonly known as ternary lithium batteries, are outcomes of this approach. The term “ternary” describes that the positive electrode of the battery comprises three elements, which are specifically chosen with an aim to reduce the cobalt content in LCO.
First, the NCA configuration is created with the addition of nickel and aluminum to LCO. Its higher nickel ratio enhances energy outputs. To put it simply, manganese in NCM is substituted with aluminum to make NCA. Unfortunately, producing NCA is more difficult than NCM since it requires more sophisticated techniques. The proprietary technologies of Samsung SDI in the NCA chemistry make it possible to produce batteries that have higher capacity and safety compared to the existing cathode materials. Based on such differentiated NCA competitiveness, we successfully verified performance and mass-production feasibility of cylindrical batteries built with the NCA cathode configuration that has more than 91% high-nickel content. This technology is also embedded in P6, our prismatic battery product for electric vehicles (EVs), which is under mass-production in 2024. What's more, by adding aluminum and employing a special coating technology, we have succeeded in minimizing battery deterioration (natural decrease of capacity and voltage upon repeated charging and discharging) to improve capacity and safety of batteries.
Next, the NCM battery is made by adding nickel and manganese to LCO. The lithium and NCM are put together at a ratio of 1:1, with the most common ratio of nickel, cobalt, and manganese in NCM being 1:1:1. Overall, we can say that the ratio of lithium, nickel, cobalt, and manganese is 3:1:1:1. As mentioned earlier, NCM was invented to maximize the content of nickel, which is cheaper than cobalt.
LMO is produced by replacing cobalt with manganese in LCO. First developed in the 1980s, its commercialization came in 1996, which is relatively late compared to LCO. The biggest benefit of LMO comes from its price stability. Unlike cobalt, which is costly and difficult to obtain, manganese is economical and its production areas are evenly distributed throughout the world. Unfortunately, what LMO lacks is the capacity comparable to LCO. This is because manganese does not directly engage in redox reactions while limiting the inflow and outflow of lithium ions. Also, it doesn't perform well under high temperatures.
LFP batteries do not use expensive cobalt and have similar advantages and disadvantages to LMO. The main reason is that iron (Fe) and phosphate (P)—the major components of LFP—are inexpensive and abundant. The characteristics of LFP are closely linked with those of iron. Iron has a very stable chemical structure, making the movement of lithium ions easy, and the required technical difficulty is relatively low. In addition, iron is economically more attractive as it is widely available across the world.
One major downside of the LFP chemistry, however, is that it has a low energy density because its average voltage is lower than LCO by at least 0.5V. It is heavy as well because of its core material, iron. Considering that electric carmakers prefer lightweight batteries with high energy capacity, the application of LFP to EVs has its limitations. To tackle this issue, price-competitive Chinese battery makers are manufacturing LFP batteries for EVs with a relatively short driving range, or for energy storage systems (ESS), as part of their attempt to bolster LFP business.
Until now, battery manufacturers are focusing on maximizing strengths and complementing weaknesses of each element. But, the pros and cons of cathode active materials are driven by core materials’ benefits and limitations, manufacturers are recently developing cathode active materials by adding new metals or adjusting their contents.
