Technology & Products
[Battery101] Inner World of Batteries
2024.06.14
|
101[wʌ́nouwʌ́n] means basic knowledge of a topic or collection of introductory materials to a topic. Our Battery 101 series talks about all things battery: the history, technical aspects (basic principles and mechanisms), industrial aspects (IT, electric vehicles, ESS, etc.), and next-generation technologies that SAMSUNG SDI will innovate while opening up its future. Batteries have infinite potentials that exceeds our wildest imagination. Through Batteries 101 series, you will have a chance to see the entire spectrum of the battery's possibilities and to conjure SAMSUNG SDI’s pivotal role in it. |
Four major components of a battery
When a primary battery cell first came out, people thought that electricity stored inside a cylinder-shape battery would power a device when connected. So some curious people considered a used-up battery as an empty can and took apart. And they even punctured it with a nail. It was reckless as people didn't know what was inside a battery.
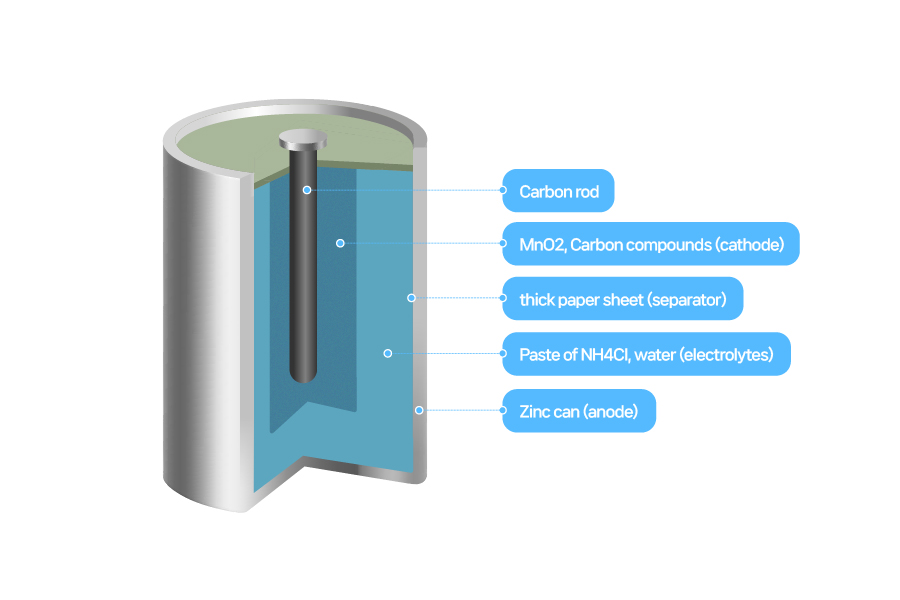
[Structure of a manganese battery]
Both primary and secondary cells comprises four major components: anode, cathode, separator and electrolyte. Electrolytes may not be easily visible as those going into a battery are usually aqueous, but it’s easy to identify with your eyes. Let's go over four key parts of a lithium-ion battery, which is a prime example of a secondary battery.
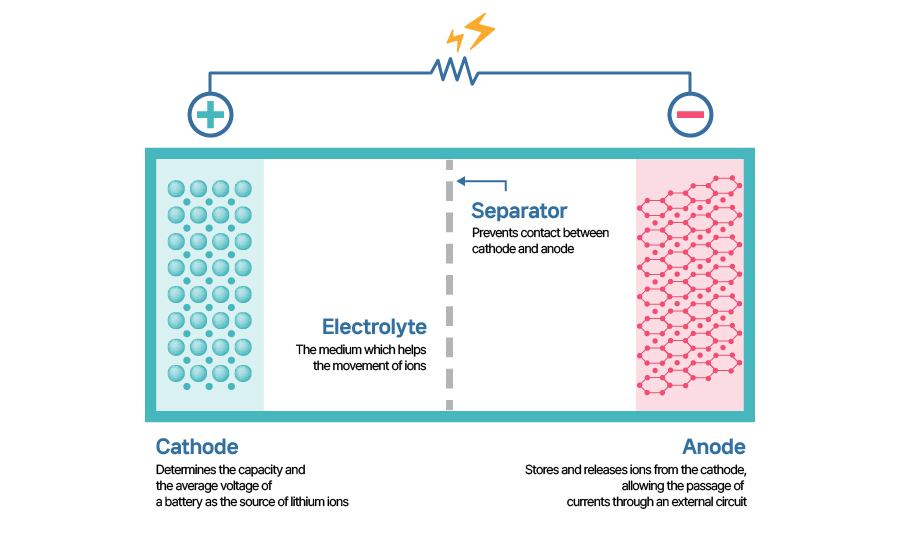
[Four components of a lithium-ion battery]
First, cathode determines the performance and specifications of a battery. When talking about a battery, the main focus is on its charge capacity and power, both of which cathode is the main determinant of. Since a battery's voltage is dependent on the electric potential difference of cathode, the electric potential value created by the respective cathode structure exerts a great influence on the voltage. The battery industry is working to develop better cathode active materials (CAM). Active materials, a defining factor of the performance of cathode, react chemically inside a battery to produce electrical energy. For a lithium-ion battery, cathode is composed of an aluminum-foil substrate as the metal part while having lithium metal oxides as the active material part.
Aluminum is served to collect electrons. When a battery is charging, electrons are released from the cathode, which would be an oxidation reaction. Upon battery discharging, the cathode receive electrons traveling from the anode – a reduction at the cathode. Here, the element involved in the redox chain reaction is not aluminum but lithium metal oxides, which are directly linked to how many volts and how much capacity a battery can have.
Anode stores the electrons and lithium-ions released from the cathode when charging and releases them back when discharging. Electrons flow through the external circuit while lithium ions move through the electrolyte to reach the cathode. At discharging, the reaction at the anode is the source of electrons for the current. Anode active materials (AAM) also contributes to capacity of a battery just as cathode active materials does. Anode has a copper-foil substrate as a current collector while using mostly graphite as its active material. Graphite expands and contracts on receiving and releasing of electrons, which affects battery life.
The separator is a physical barrier between the cathode and the anode. If the cathode and anode should come into contact with each other, it will short out the battery, generating heat rather than electricity and eventually causing sparks. Because lithium itself is highly reactive, lithium-ion batteries pose a higher risk in that regard. That’s where separators come into play to prevent such unfortunate incidence from happening and ensure battery safety. They should be able to electrically insulate the both electrodes, be thermally stable, and automatically shut down the movement of ions if temperatures rise above a certain level.
The electrolyte allows ions to be permeable yet impermeable for electrons so that the electrons only move through the external circuit. They also should have high levels of ionic conductivity, electrochemical stability, and ignition point for the sake of safety. To be a strong ionic conductor, electrolytes are usually made of liquids, with the addition of polymers or ceramics to increase the level of viscosity, which improves safety of the electrolyte. However, additional process means higher cost increase to make them.
Li-ions and electrons move to generate electricity
People say secondary batteries are economical and eco-friendly because they are rechargeable and power battery-electric vehicles which have significantly lower carbon emissions compared to internal combustion engine cars. That’s why the invention of rechargeable batteries and their evolution to boast higher capacity and output marks a very meaningful milestone for the mankind. Here’s a visualization of the flow of ions during charging and discharging.
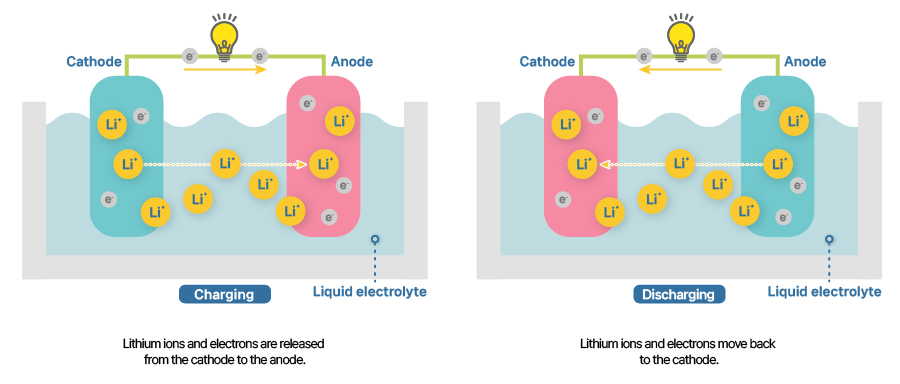
[Lithium-ion battery’s charging and discharging]
When a secondary cell is charging, oxidation occurs at the cathode while reduction occurs at the anode. Electrons are represented as grey balls in the picture above, leaving the cathode for the anode through the circuit whereas lithium ions, pink balls, move towards the anode via the electrolyte. And the reverse happens when discharging.
At a normal state, a metal’s atom doesn’t release electrons, which means it stays neutral, without any charge. When a metal is placed in a chemical cell, the difference of the ionization energy between the cathode and anode materials causes the metal to lose electrons (negative charge) and form positively charged ions. These electrons and ions migrate towards the same destination, either the anode or cathode.
We use a smartphone for at least a couple of years without replacing a battery. A couple of years because it happens to be the approximated period when a battery reaches 80% of its original capacity under normal usage conditions. Many people do not even bother replacing a battery or a smartphone because a device with lower than 80% battery health doesn’t even inconvenience the device experience too much. Relentless efforts in researching and developing longer and stronger batteries make such device experience possible.
