Technology & Products
[Battery101] Difference between Primary Cells and Secondary Cells
2024.05.24
|
101[wʌ́nouwʌ́n] means basic knowledge of a topic or collection of introductory materials to a topic. Our Battery 101 series talks about all things battery: the history, technical aspects (basic principles and mechanisms), industrial aspects (IT, electric vehicles, ESS, etc.), and next-generation technologies that SAMSUNG SDI will innovate while opening up its future. Batteries have infinite potentials that exceeds our wildest imagination. Through Batteries 101 series, you will have a chance to see the entire spectrum of the battery's possibilities and to conjure SAMSUNG SDI’s pivotal role in it. |
Primary cells cannot be recharged
The difference between primary and secondary cells can be summed up in one word: reusability. Primary cells, once used, cannot be reused and secondary cells can for many times. Most batteries we see and use are chemical cells, which generate energy from changes of matter. That is to say that chemical cells convert chemical energy into electrical energy using oxidation and reduction reactions, also known as redox reactions.
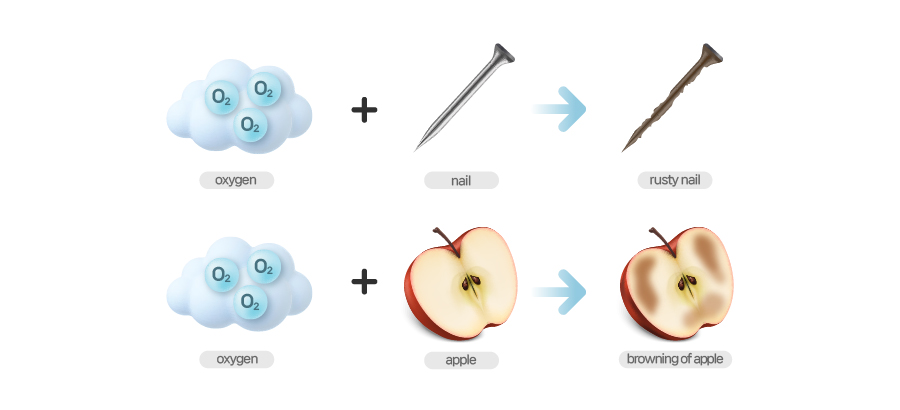
[Oxidation]
Oxidation occurs when bonding with oxygen forms whereas reduction occurs when bonding with oxygen breaks. Let me give you an easy example. When you left apple slices in room temperature, those apple slices turn brown. The same goes for rusty nails. Over time, a shiny nail and shovel gets rusty. What triggers such change in color and texture of apple slices and metal is air. To be more specific, it is oxygen in the air that does this. When oxygen bonds with an atom or a compound, it triggers a chemical reaction like browning or rusting.
Oxygen is atomic number 8 which means it has 8 protons. But it has 6 valence electrons which are very unstable because atoms stay stable when they have 8 electrons in their valence shells. So those 6 valence shells are most eager to take 2 more electrons from somewhere else. This is what prompts oxygen to steal electrons when bonding with other matter and change the chemical properties.
As scientists investigated redox reactions triggered by oxygen, they found that these chains of chemical reactions occur even in the absence of oxygen. Since they couldn’t keep coming up with new names for each of those reactions, they came to an agreement that any type of reactions where loss of electrons happens was to be called oxidation, and for gain of electrons, it would be called reduction. This is how we can explain all electron transfers through the reduction-oxidation mechanism.
Chemical cells change chemical energy into electrical energy through transfers of electrons and use metal as the key materials. Non-metals act as an oxidizing agent, gaining electrons, and metals are a reducing agent, losing electrons. For instance, primary cells use metals for the positive electrode (cathode) and the negative electrode (anode). The basic principle is that metals of a stronger reducing agent are used for the anode, and the ones of a stronger oxidizing agent is used for the cathode.
We now go back to the beginning. Let me explain why primary cells can’t be recharged. First, there is no such thing as “charging” in the making of primary cells. It cannot be charged once for a single use. Then another question comes, “How come we can use batteries that are not even charged?”
Primary cells use electricity generated by the electromechanical reaction in the battery, where electrons released by the anode move to the cathode. This process is called discharge. When discharging primary cells, electrolyte change occurs in both electrodes. Namely, metal bonding undergoes structure change which then leads to change in properties and shape, not to mention electrolyte composition. Such changes are irreversible, as you might see from apple that turned brown or rusty nails won’t go back to its original state. The entire process of discharging in primary cells, as such, is not reversible. This is a key feature that distinguishes primary cells from secondary cells since in secondary cells, the chemical reactions are indeed reversible. So it makes primary cells unusable even if they go through charging process again.
But what would happen if someone mistakes a primary cell for a secondary cell and plug it into a charger? Let’s say we connect power to the both ends of electrodes on an alkaline manganese battery and try charging. Within minutes, we won’t notice much change. But it becomes a different story when left for a long time. Flow of electricity in a discharged battery will spark electrolysis of water in electrolytes. Due to different compositions of the anode and cathode, hydrogen gas forms in the cathode and oxygen gas forms in the anode, which can cause a big explosion if mixed. That is why you see a warning label on alkaline manganese batteries that say, “Do not charge.” You should never charge primary cell batteries, or connect electricity through these batteries, since it will generate gas.
Secondary cells can be chargeable
Let’s find out why secondary cells can be chargeable, starting with lead-acid batteries.
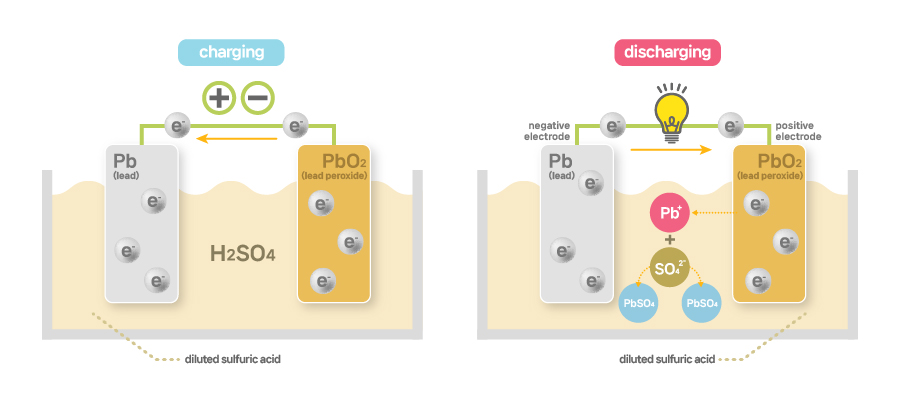
[Mechanism of lead-acid batteries]
Fully charged lead-acid batteries have the positive plate of lead peroxide, negative plate of lead and electrolytes consisting of water and diluted sulfuric acid. During discharging, those lead peroxide and lead on the plates turn into lead sulfate, reducing sulfuric acid in the electrolytes and increasing the water content. That is, if fully charged, sulfuric acid on the plate will decrease, increasing the electrolytes’ sulfuric acid content, which prompts lead peroxide to move back to the positive plate and lead to the negative plate, rendering the electrolytes aqueous sulfuric acid. Put in other words, in lead-acid batteries, discharging brings about oxidation in the negative plate and reduction in the positive plate, and vice versa in charging. This process is all reversible, allowing for repeated charging and discharging.
As stated above, since its invention in 1859, the lead-acid batteries have been in use as automotive batteries. This tells us that there are an abundance of advantages of lead-acid batteries. One big advantage is their price. Because lead-acid batteries use nonferrous lead, they are cheaper than using zinc, about one fifth to one tenth of the price for nickel, making it much easier to source the materials. The simple structure of the battery means easy maintenance as well. In addition, they can be discharged over a large range of temperature and also can be discharged over a short period of time releasing strong electric current.
Lastly, they can be equipped with large capacity, which means they can come in a variety of sizes. 450 units of lead-acid batteries with dimensions of 30cm×30cm×150cm built into a submarine work as a sensitivity adjustment screw. Versatility at its working, wouldn’t you say?
But the disadvantages are shorter life cycle and low energy density. Automotive lead-acid batteries look like a big box where six cells with 2.1 voltage are linked together inside. You need to connect them in series to configure required voltage of 12v to 13v. Besides, they are prone to self-discharging, difficult to put in long-term storage after discharging, primarily made of lead, which is a heavy metal, and need caution for fire that can be caused by hydrogen gas formation, and the list goes on. Resultingly, the era of lead-acid batteries has come to a stagnant state since 2010 with the release of new types of secondary batteries.
Lower price and higher performance
Recently, rechargeable secondary cells are in the spotlight as a solution to environmental issues. Latest trends in the secondary battery market are smaller size and larger capacity, garnering more and more attention as the “future-leading industry.” Let’s take a closer look at the history.
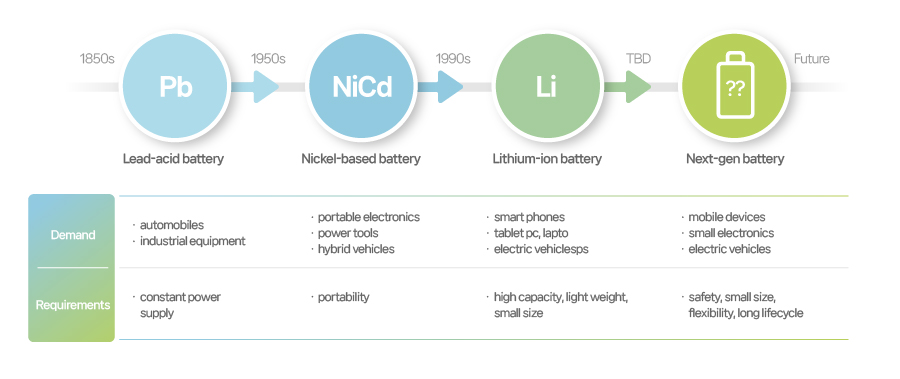
[History of secondary development]
Lead-acid batteries we talked about earlier can be recharged but its big size and short life cycle prevented them from being the industry-prevalent battery. Their main use was limited to automotive battery and auxiliary device for operating industrial equipment. Many researchers, however, were inspired by this limit and perceived the need for new types of secondary batteries without lead-acid battery’s cons and with added pros. In this sense, lead-acid batteries was a driver in leading the growth of the secondary battery industry.
Ni-Cad batteries are sturdier, stronger to hold against impact, and can be sealed, applying to a wide range of portable devices like trimmers, mobile phones, and power tools. With the rise of portable device market, Ni-Cad batteries became the dominant force in a new battery market. However, it suffered from a “memory effect” where the capacity drops if they are recharged without being fully discharged and customers started walking away from them. The use of cadmium, which can cause itai-itai disease, was problematic as well.
To solve the issues posed by nickel-cadmium batteries, nickel-hydrogen batteries came out in 1990. They had twice as the capacity as Ni-Cad batteries and much higher safety, making them a popular adoption for new dawn of electronic devices like mobile phones and laptops. But with a new wave of lithium-ion batteries in mid 1990s, the rise of nickel-hydrogen battery was halted. While lithium-ion batteries were expanding its market presence with key features of high capacity and long battery life, nickel-hydrogen battery didn’t have much improvement in enhancing energy density.
Li-ion batteries, the predominant contender in the current secondary battery industry, had its debut in early 1990s. One lithium-ion battery holds a capacity that is equivalent to three nickel-based batteries, most suitable for portable electronics. Moreover, it does not have the memory effect even after intermittent and repeated charging cycles which serve to add to performance of mobile phones and laptops. These edges that lithium-ion batteries boast eventually turned Li-ion batteries from high-end applications in mid 1990s to wide, general applications by 2000s.
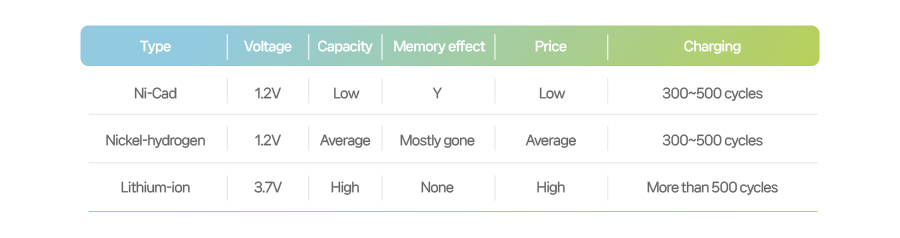
[Types of chemical cell]
Constant downward trends in the price propelled the spread of Li-ion battery adoptions. According to a survey by Bloomberg, the price of a lithium-ion battery pack per kWh came down to 139 US dollars in 2023, a drastic drop by 82% in ten years compared to the price recorded in 2013, which was 780 US dollars.
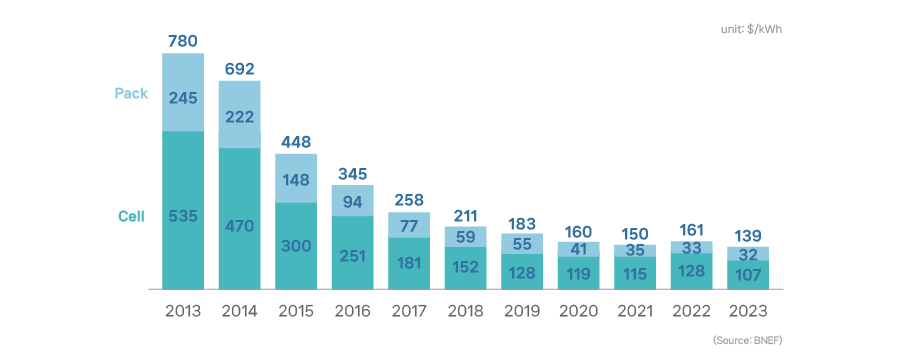
[Changes in lithium-ion battery price]
