Technology & Products
[Battery101] History of Battery
2024.05.17
|
101[wʌ́nouwʌ́n] means basic knowledge of a topic or collection of introductory materials to a topic. Our Battery 101 series talks about all things battery: the history, technical aspects (basic principles and mechanisms), industrial aspects (IT, electric vehicles, ESS, etc.), and next-generation technologies that SAMSUNG SDI will innovate while opening up its future. Batteries have infinite potentials that exceeds our wildest imagination. Through Batteries 101 series, you will have a chance to see the entire spectrum of the battery's possibilities and to conjure SAMSUNG SDI’s pivotal role in it. |
The first-ever battery was made 2000 years ago?
The voltaic pile that we know to be the first true form of a battery was invented in 1800. Does that mean the battery has just about 200 years of history? Surprisingly, that is not the case. The first time a battery is estimated to have emerged is around 3rd century BC to 7th century AD. This estimation was possible because of the most surprising discovery of all happened in the summer of 1932, in Iraq. When archaeologists at the Iraq Museum was undertaking an excavation of ancient ruins near Baghdad, they uncovered a set of terracotta pots approximately 14-cm tall, with a peculiar shape.
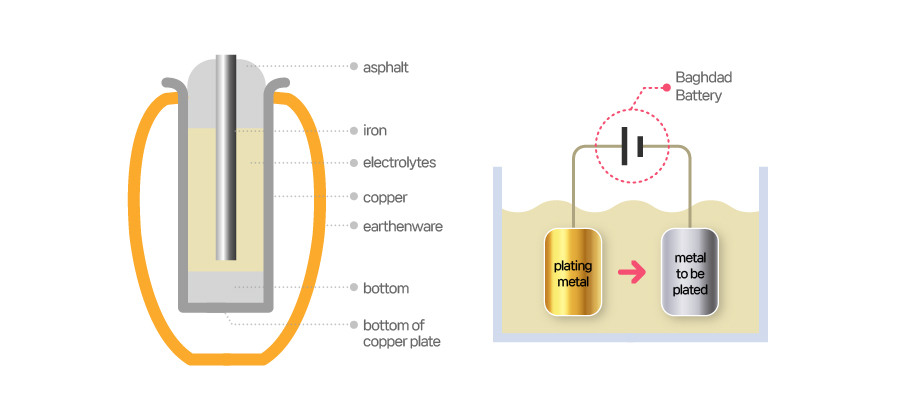
[Mockup design of Baghdad Battery]
The pot was rather small, about an adult's handful size. If you looked at the mouth, you would notice something unusual, though. It contained an iron rod which was 1 cm in diameter and a copper sheet rolled around it. The mouth was covered tight with dark brown asphalt, or natural oil tar. Archaeologists got rid of brown tar in the mouth to find out its purpose. The pot was 2.6 cm in diameter and 10 cm length and contained an iron rod which was 1 cm diameter and 7.5 cm in length.
It was Wilhelm König, a curator of artifacts at the National Museum of Iraq, who noticed that the structure of the jar was very similar to that of a battery because He came up with this idea because the excavated iron rod was rather unusual. An iron rod that's been buried for decades is bound to get rust. But the iron rod in the jar seemed like it was corroded by acid.
In the 1930s, it was already known that batteries consist of anodes, cathodes, and electrolytes. It was also likely that people understood that electricity is generated by the potential difference (difference in electrical potential energy) between the two metals, which is the basic principle of batteries. With such knowledge, König concluded that the jar worked as a battery of some sort and named it Baghdad Battery. Subsequent research papers were published as well. König argued that the excavated area was the ruins of the Parthian empire and that the battery also came during that empire's time.
So, what did people use this Baghdad battery for? Researchers presume that it was used for plating or for invasive or magical purposes to relieve pain. Plating is a more prevalent theory. Scientists carried out experiments to see if it really worked. When they used a copper plate as an anode, the iron rod as a cathode, and vinegar or wine as an electrolyte, it was indeed confirmed that electricity current was created. Plating with such electricity is called electroplating. When an object to be plated is immersed in a plating solution and DC current is sent through, a thin metal coating is formed on the surface, which can be used to plate silver or gold.
Origins in jewels and artillery corps
When did humans start noticing the phenomenon of electricity? A popular gem stone was something called "electron," currently known as amber. Amber is a yellowish organic substance made when pine resin is fossilized, which makes it inflammable, uncharacteristic of usual gem stones. When rubbed with fur, static electricity is generated to attract nearby light objects. We can only assume that those Ancient Greeks felt that static electricity when they were rubbing something with amber.
It remained a 2000-year-old mystery as to why such phenomenon was sparked with amber, of all materials. By the 16th century, an English physicist William Gilbert who had been working on electricity and magnetism first used the word "electricus," meaning "like amber" and that led to the new modern word "electricity." In the end, it was all about amber when it came to the etymology of the vocabulary relating to electricity.
Moving onto the etymology of the word "battery."
It was Stephen Gray who first discovered conductors and non-conductors in 1729. He demonstrated that electricity would travel through wires. In 1746, a Dutch scientist Pieter van Musschenbroek invented “a jar that stores an electric charge” and conjured a practical concept of a battery. That bottle created by Musschenbroek was named Leyden jar. Leyden jar is a glass jar covered with metal foil inside and out. It would have an insulator, such as cork, as its lid and a metal terminal and chain projecting through that lid to reach the bottom of the jar.
The mechanism of Leyden jar to collect electricity is rather simple. You can just think of an elementary school experiment where you rub a fabric cloth on a glass rod to generate static electricity. But when you take that glass rod to a metal rod right after rubbing it with a fabric cloth what happens is that generated static electricity travels through the metal rod and to the tin foil on the inner surface. This is how Leyden jar captures static electricity to work as a battery.
To put it simply, the positive charge on the glass rod moves through the metal terminal and chain to enter the jar. It creates a positive charge in the tin foil on the inside surface of the jar and a negative charge in the tin foil on the outside surface. The opposite charges attract each other with the jar in between, basically being stuck there and stored in Leyden jar.
This invention of Leyden jar was such a sensation back then. People could actually feel electricity when a conductor came in contact with the metal terminal projecting through a Leyden jar. Many scientists threw themselves to make their own Leyden jar. One of them was Benjamin Franklin, whose face is on the 100 US dollar bill, and who is known to have invented the lightning rod. Upon hearing the news of a Leyden jar in Europe, Benjamin Franklin participated in a reproduced experiment himself and embarked on the research on the electricity. In 1752, when it was raining, he went out in the search for lightning with a Leyden jar in his hand. When he finally managed to encounter lightning, he proved that lightning was in fact electricity by demonstrating that it charged the Leyden jar. This led to the invention of a lightning rod which transfers electric energy from a lightning strike into the underground.
Benjamin Franklin was the first person ever to use the term “battery”, to describe a power supply device, or a series of similar objects grouped together to perform a function. He called this series of Leyden jars linked together a battery, which to people back then would usually mean an artillery subunit of soldiers. Franklin thought that this joined Leyden jars served the same purpose, much like a battery. Later on, the word "battery" gained an additional definition to mean a container used as a source of power.
Voltaic battery, the original form of batteries
In 1786, Luigi Galvani, an Italian lecturer in biology and anatomy at the University of Bologna, discovered that legs of a dead frog on a copper plate twitched when touched by a knife. The same happened when he wired a dead frog on a copper wire and touched it with a knife. Based on these astonishing experiments, he published a paper claiming that there is a life source called "animal electricity," which activates when muscles or nerves get stimulated by metal.
When this paper titled Commentary on the Effect of Electricity on Muscular Motion was released in 1791, many chimed in to conduct frog experiments. One of them was Alessandro Volta, also known as the father of batteries. Despite not being educated at a university level, his reputation in the electricity academia was already exceptional. At his young age, he became a professor of experimental physics at a university and that was when he encountered the Galvani's famous paper and started to doubt.
When Volta conducted the experiment with iron plates, not copper plates, the frog's legs didn't move. This led Volta to think, 'The current flowing through the frog's legs is not necessarily animal electricity, but perhaps by different metals?' To prove this hypothesis, he made his own battery, which is the famously known Voltaic pile (Voltaic cell).
Volta batteries are stacked with zinc, silver (or copper) and paper soaked in salt water. It took in the principle of Galvani's experiment: two different metals and frog's body fluid. Volta confirmed that the electricity current flowed in his model. This is the origin of a battery using metals and electrolytes that are used to this day. Volta's experiments also showed that electricity requires materials that act as anode, cathode, and electrolytes.
Evolution of batteries
Volta's cells were heavy and huge because it had piles of anodes, cathodes and sheets of salt-watered paper. Its sudden drop in power stopped it from becoming an industry-level development. It was English physicist and chemist John Frederic Daniell who complemented Volta pile's shortcomings and made a successful commercial product out of chemical batteries. Daniell cells were invented 20 years after the invention of Volta cells. The same metal was used for the anode and cathode and there was a barrier separating them, using different electrolytes for the anode and cathode. A zinc sulfate solution was used for the cathode, a copper sulfate solution was used for the anode, and two electrolytes were separated by a salt bridge. This salt bridge prevented electrolytes from mixing and acted as a path for ions to move smoothly through chemical reactions. Thanks to this, batteries with longer life came out. A Daniel cell generated 1.1 volt electricity and was initially used to power communication facilities.
The next breakthrough was the invention of rechargeable lead-acid cells. The previous versions of batteries were primary batteries which got discharged permanently, without being able to recharge, once all chemical reactions were finished. In 1859, French physicist Gaston Planté discovered that passing a current through dissolved sulfuric acid with lead in it made charging and discharging of a battery. This mechanism is in use to date, namely in lead-acid batteries (also known as Planté Battery). An advent of rechargeable battery at last! The first version of a lead-acid battery had lead dioxide on the positive plate, lead on the negative plate, and dissolved sulfuric acid as electrolytes. Lead-acid batteries are still widely in use and are built into all internal combustion engines of vehicles as a start engine battery.
In 1870, French chemist Georges Leclanché made 1.5-volt cells but they were not ready for portable use due to the limitations of wet cells and issues involving leaks and corrosion. Then came Carl Gassner, a German doctor and inventor, to solve the problem. He added gypsum plaster to electrolytes which he patented in 1886 in Germany and then in 1887 in the United States. By 1896, mass production of dry cells started. That’s how dry cells came to be known.
Nickel-cadmium batteries were invented in 1899 by Waldemar Jungner, a Swedish inventor. Ni-cad batteries featured higher energy density than lead-acid ones. And then Thomas Edison released a ground breaking invention. He revised the design of the nickel-cadmium battery to invent nickel-iron batteries by having an electrolyte of potassium hydroxide. The reason Edison worked on making batteries was that he wanted his batteries to be adopted by electric vehicles. However, as the demand for the EVs decreased, nickel-iron batteries went into industrial and railroad works.
Invented in 1955, alkaline batteries have the longest history among any other batteries still in use. Before alkaline batteries, zinc-carbon batteries were popular. However, they had low energy and short life span. Many researchers conducted research to address this issue. Lewis Urry working at Eveready Battery Company was one of those researchers. He discovered that switching electrolytes from acidic ammonium chloride to the alkaline potassium hydroxide worked well to increase the battery longevity and invented an alkaline battery. Because alkaline corrodes metals much slower than the acid does, batteries with alkaline electrolyte would have longer life, for which they are still very much loved.
