Technology & Products
[Battery101] Electrolyte, the Best Driver for Lithium Ions
2024.07.19
|
101[wʌ́nouwʌ́n] means basic knowledge of a topic or collection of introductory materials to a topic. Our Battery 101 series talks about all things battery: the history, technical aspects (basic principles and mechanisms), industrial aspects (IT, electric vehicles, ESS, etc.), and next-generation technologies that SAMSUNG SDI will innovate while opening up its future. Batteries have infinite potentials that exceeds our wildest imagination. Through Batteries 101 series, you will have a chance to see the entire spectrum of the battery's possibilities and to conjure SAMSUNG SDI’s pivotal role in it. |
The electrolyte guarantees the safe and fast lithium ions transport
The electrolyte is a substance that allows lithium ions to travel between the cathode and anode freely. It stabilizes cathode and anode surfaces, extends the lifespan, and improves the characteristics of the battery as well.
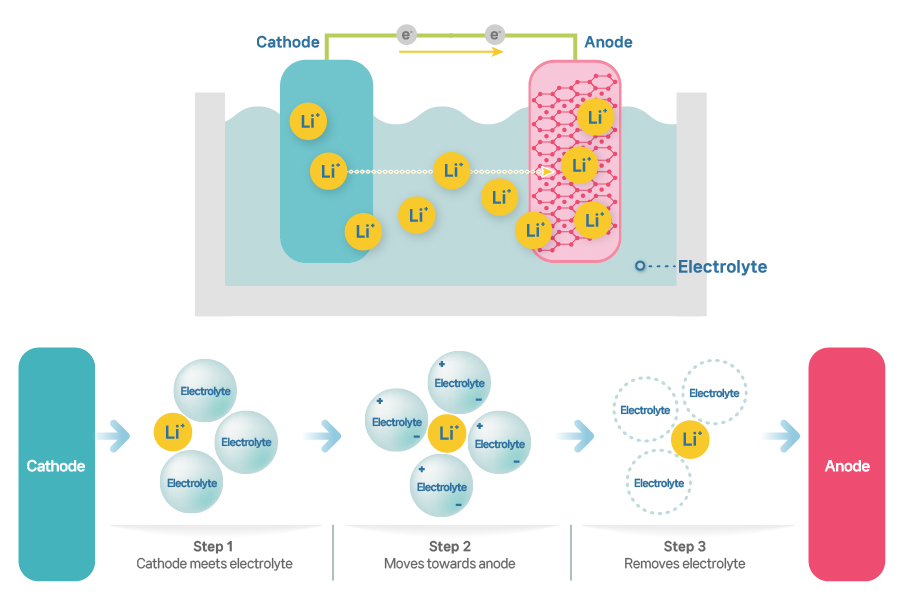
[The role of electrolytes in lithium-ion batteries]
Today, lithium-ion batteries mostly use liquid electrolytes (an electrolyte solution). A liquid electrolyte is a lithium salt dissolved in an organic solvent. Ionized lithium ions move towards the anode through the electrolyte during charging, and these lithium ions settle in the anode material (graphite). Looking closer, you will find out that lithium ions in the electrolyte serve as a kind of passage that allows lithium ions to move from cathode to anode. You might wonder why it’s “a kind of passage”, rather than just a “passage”.
In fact, many pictures illustrating the working principle of lithium-ion batteries have an error. They depict lithium ions swimming in the electrolyte. To put it bluntly, lithium ions released from the cathode active material do not “swim” to move towards graphite anode. Rather, they are carried.
Let me explain the charging process of a battery. Lithium ions released from the lithium metal oxide (cathode) enter the electrolyte during charging. There are already plenty of lithium ions dissolved in the lithium salt. The lithium ions released from the lithium metal oxide push the lithium ions dissolved in the electrolyte to the side. Again, these lithium ions push the neighboring lithium ions until the lithium ions right in front of the anode active material enter the graphite layer.
The vice versa takes place during discharging. When the lithium ions in the graphite layer are released into the electrolyte, they keep being pushed to the next until they return to the lithium metal oxide.
An organic solvent, the main component of the electrolyte, is flammable, posing a risk of ignition. This is why battery manufacturers ardently research solid electrolytes to solve this problem. We will dive into all solid-state batteries later.
The three elements of electrolyte: Lithium salt, organic solvent, and additives
An electrolyte comprises a lithium salt, an organic solvent, and additives. Imagine a cup of coffee: water (an organic solvent) with melted coffee powder (a lithium salt) and added sugar (an additive). These three components have their unique roles and functions just like coffee, water, and sugar do in a cup of coffee.
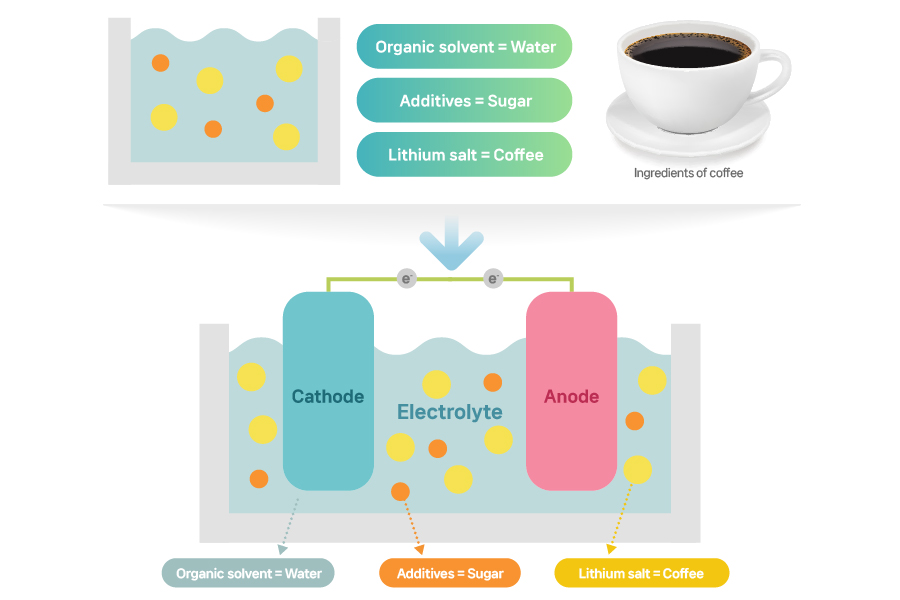
[The composition and roles of the electrolyte in lithium-ion batteries]
The more lithium ions there are, the better electrolytes work. This is also why battery makers choose a lithium salt with good ability to dissociate lithium ions. The most commonly used lithium salt is LiPF6 (lithium, phosphate, and fluorine), which facilitates more efficient ion transport, dissolution, and higher chemical stability than other salts.
An organic solvent works to dissolve a lithium salt. We use an organic solvent rather than water because lithium is a metal that is highly reactive when it comes in contact with water. An organic solvent doesn’t dissolve in water well, is highly volatile, cleans well, and comes with peculiar odors. Dry cleaning fluids used in washing some of clothing items, acetone that acts as nail polish remover, and ethanol used as a perfume solvent are all organic solvents.
As the organic solvent in electrolytes works to dissolves lithium salt, it facilitates the travel of lithium ions. To make this happen, the organic solvent must meet a few requirements. First, the organic solvent should have high solubility for lithium salts to separate ionic compounds. Second, it should have a low viscosity for seamless movement of lithium ions. Although it varies from solvent to solvent, in order to have high ionic conductivity (efficiency of ion transport), a mixture of solvents is used. Third, the organic solvent should have low chemical reactivity. This is crucial because it may cause safety issues when a solvent reacts with the cathode and anode.
Additives form a protective film on the cathode and anode surfaces. They enable smoother movement of lithium ions between the cathode and anode. They also prevent battery degradation. Because lithium-ion batteries exhibit poor stability during charging and discharging, we use a small amount of additives to improve battery performance and safety.
Additives are further divided into cathode and anode additives. Cathode additives stabilize a cathode structure and protect the surface to prevent battery aging, thus reducing the risk of overheating and overcharging. Anode additives are dissolved before a solvent is, forming a strong layer in the anode to increase a lifespan. Also, anode additives prevent overheating and maintain battery capacity, while reducing internal gas generation. As a rule, electrolyte additives for both cathode and anode should dissolve well in liquid electrolytes and be chemically stable.
Since batteries with higher voltages are in growing demand these days, battery companies are developing additives that can protect both cathode and anode at high voltages. There are also additives that help detection of defects, preventing contamination during battery production. In short, though additives make up only a small portion in liquid electrolytes, they play a holistic range of crucial roles in electrolytes such as improving a lifespan, addressing high-temperature issues, and reducing resistance.
